Our FDA & EMA Orphan Drug Designation Services
Only Orphan Cote provides you with an array of best-in-class services to guide you through FDA and EMA orphan drug designation for your rare disease assets.
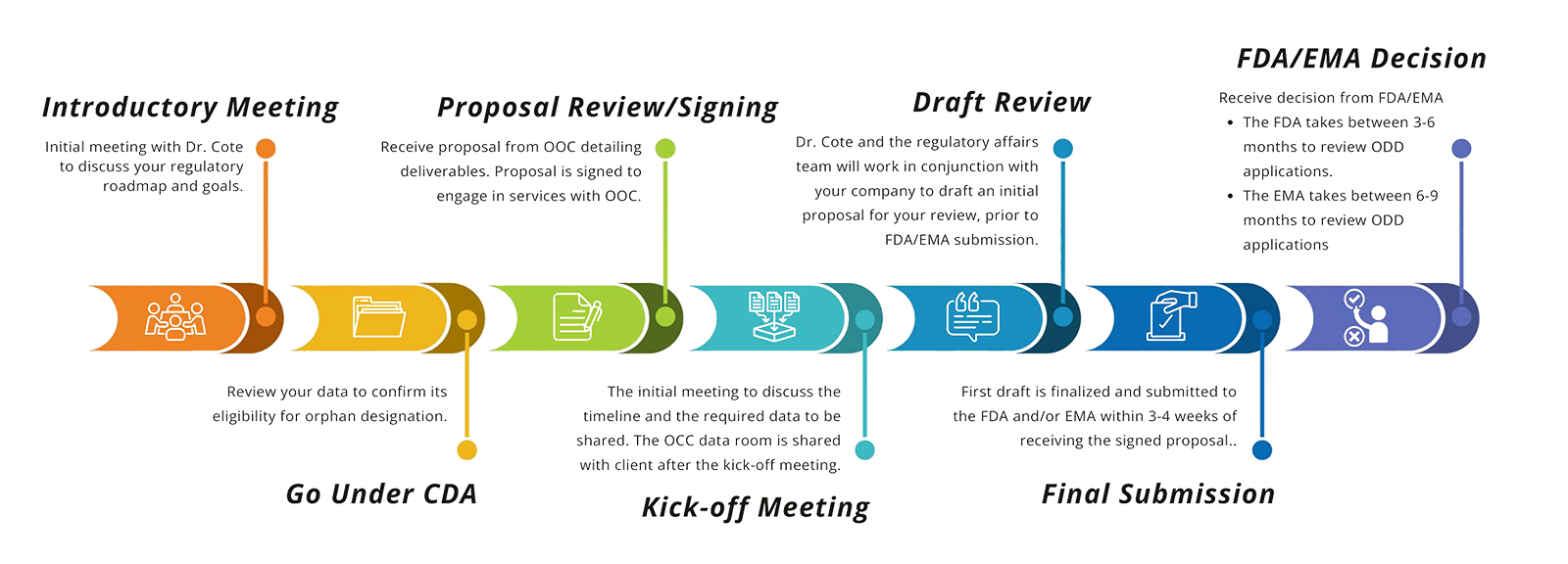
Our FDA Orphan Drug Designation Process
Meetings with the FDA
(INTERACT, Pre-IND, Type A, B, C, D)
From the pre-IND meeting to the pre-NDA/-BLA, you will meet with the Agency to gain insight into their thinking about your drug development. We know how to optimize these meetings so that you can get the most out of FDA resources that are accessible to drug developers. Learn more.


Submissions for FDA Expedited Programs
• Breakthrough Therapy Designation
• Fast Track Designation
• Accelerated Approval
The FDA’s expedited programs are designed to accelerate the development of certain drugs. Breakthrough Therapy and Fast Track both require a designation to obtain their incentives. Only Orphans Cote will guide you through every step.
Accelerated Approval, on the other hand, allows a sponsor to gain approval on a surrogate endpoint. We provide expert support in preparing for FDA meetings and building a strong case to use a surrogate endpoint, helping you move toward approval faster.
Learn more about our
FDA Expedited Program Solutions.
IND Applications
Prior to first-in-human studies, you must file an Investigational New Drug (IND) application with the FDA to demonstrate that it is safe to use your drug. After being informed by a pre-IND meeting, we will review, analyze, and write your IND for application for submission to the Agency.


Regulatory Strategy
No matter where you are in your orphan drug development process, we can assess your program and bring a sound regulatory strategy to help expedite achieving your goals and ensure you are using all the regulatory perks at your disposal.
OOPD Grants Program
The OOPD Clinical Trials Grants Program, formerly overseen by Dr. Cote, is designed to advance clinical trials for rare diseases. Eligible applicants must work with a rare disease and have an active IND at the time of grant receipt. Open to public, private, nonprofit, and for-profit organizations worldwide, the program offers a unique benefit: applications are reviewed by the FDA's approval division, providing alignment and valuable regulatory insights.
In addition Only Orphans Cote supports other Orphan Products Grants:
• Natural History Studies Grants Program
• FDA Rare Neurodegenerative Disease Grant Program
Only Orphans Cote writes the grand application in a turnkey fashion. Please note that we will accept projects for Grant Submission writing no later than 4 months before the annual deadline.


Additional Services and Programs
• Qualified Infectious Disease Program (QIDP) Designation
• 505(b)(2)
Start your FDA & EMA orphan drug designation journey today.
The experts at Only Orphans Cote are here to guide you to success.
Every orphan drug development path is unique. Share your vision and needs with us, and we'll craft the right path forward.

