FDA Orphan Drug Designation Consulting
Securing Orphan Drug Designation (ODD) from the FDA is a critical milestone for rare disease therapeutics. At Only Orphans Cote, FDA Orphan Drug Designation is our flagship service.
Trusted Authority in FDA & EMA Orphan Drug Designation
95% First-Attempt Application Success Rate
Expert Guidance from the Former FDA Director of Orphan Products
Unmatched FDA Orphan Drug Application Success
Comprehensive FDA ODD Application Support
Securing orphan drug designation (ODD) requires a thorough understanding of FDA expectations, as well as precise preparation of supporting documentation. At Only Orphans Cote, we provide end-to-end support tailored to your program’s needs, including:
- Strategic Assessment – Evaluating the strength of your data package and alignment with ODD criteria.
- Regulatory Guidance – Advising on requirements, timelines, and regional differences between FDA and EMA submissions.
- Application Development – Drafting, structuring, and refining ODD applications with clarity and compliance in mind.
- Data Presentation – Organizing preclinical and clinical evidence to highlight therapeutic promise for rare disease treatment.
- Submission Support – Managing the submission process and responding to regulatory queries.
- ODD Maintenance – Providing continued guidance and FDA task implementation to ensure designation is maintained.

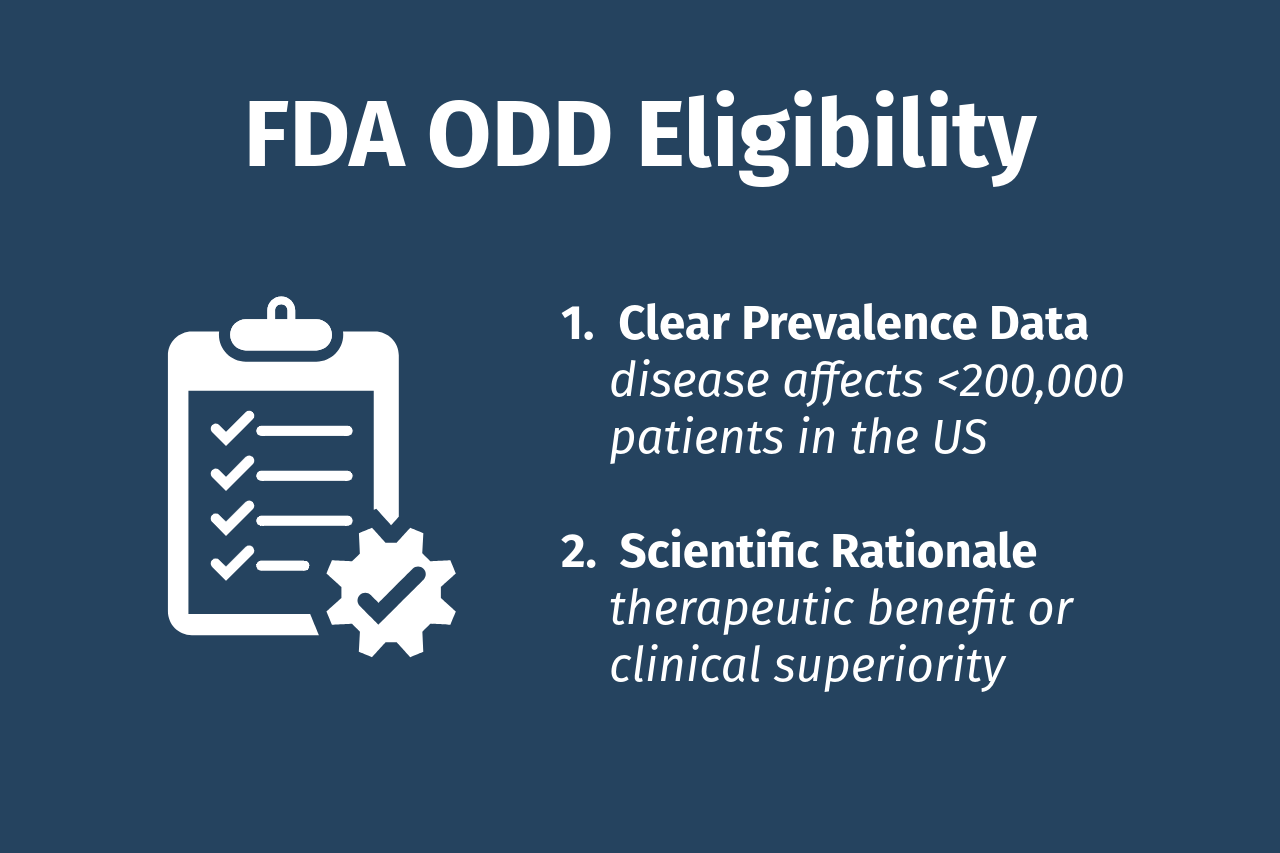
Eligibility & Requirements
- Clear prevalence data: showing the disease affects fewer than 200,000 patients in the US.
- Scientific rationale: demonstrating therapeutic benefit or clinical superiority.
- Robust preclinical data: from a well-established animal model with statistically significant results.
OR
- Clinical case data:
at least three strong before-and-after patient cases if available.
Why Biotechs Choose Only Orphans Cote
Our team has supported over 150 biotech and pharmaceutical companies worldwide in achieving ODD since 2021, enabling them to attract investment, advance clinical programs, and ultimately bring treatments to rare disease patients in need.

What is FDA Orphan Drug Designation?
The FDA grants Orphan Drug Designation to drugs or biologics intended to treat diseases affecting fewer than 200,000 people in the United States. This designation is an economic entitlement that provides critical benefits for rare disease programs.
Ready to Secure FDA Orphan Drug Designation?
We offer a free initial data review to assess ODD readiness. Our team will advise whether your program qualifies before you invest in a full application. Then, we’ll design the clearest path to help your program win FDA designation.

