EMA Orphan Drug Designation Consulting
Securing EMA Orphan Drug Designation (ODD) is a critical milestone for rare disease therapeutics. Get EMA ODD assistance from the experts at Only Orphans Cote today.
Trusted Authority in FDA & EMA Orphan Drug Designation
98% First-Attempt EMA Application Success Rate
Expert Guidance from the Former FDA Director of Orphan Products
What is EMA Orphan Drug Designation?
Typical EMA Designation Timeline
- Application review
without pre-submission meeting: ~8 months
- Application review with pre-submission meeting: ~10 months
Our Comprehensive EMA ODD Support
Our clients have secured EMA ODD across multiple therapeutic areas, enabling access to critical regulatory incentives and solidifying their long-term rare disease development strategies.
Common EMA Challenges & Our Solutions:
Navigating Complex EU Prevalence Requirements
We guide sponsors in preparing robust epidemiology data.
Expressing Significant Benefit Demonstration
We help articulate therapeutic advantage over existing treatments.
EMA Annual Reporting
We manage yearly submissions, ensuring ongoing compliance.

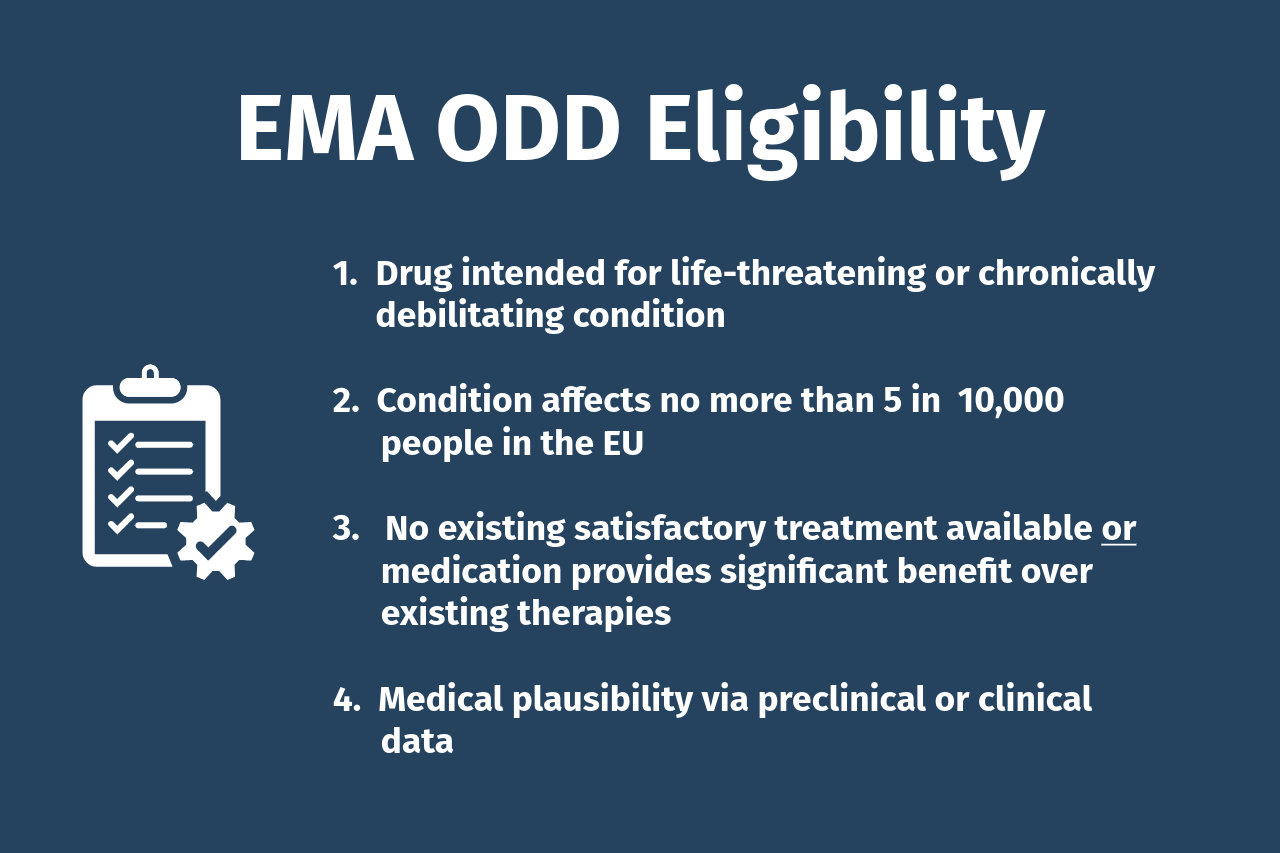
EMA Eligibility & Requirements
- Life-threatening or chronically debilitating condition: The medicine must be intended for a condition that is serious, rare, and has a significant impact on patients.
- Prevalence threshold: The condition must not affect more than 5 in 10,000 people in the European Union at the time of application, or the product must be unlikely to generate sufficient return on investment without incentives.
- No satisfactory treatment available: Either no approved treatment method exists, or the medicine will provide a significant benefit over existing therapies (e.g., improved efficacy, safety, or major contribution to patient care).
- Medical plausibility: Preclinical or clinical data must show a reasonable basis that the medicine could diagnose, prevent, or treat the condition.
Ready to Secure EMA Orphan Drug Designation?
We offer a free initial data review to evaluate EMA orphan designation readiness. Our team will advise whether your program qualifies before you invest in a full application. Then, we’ll design the clearest path to help your program win EMA designation.

